Case of the month August 2024 – Acute septic shock and progressive liver failure following high dose chemotherapy

34th ISUOG World Congress on Ultrasound in Obstetrics and Gynecology 2024
July 23, 2024
WHO: Global Plan of Action consultation (online, 20 August, 14:00 CET)
August 15, 2024Marco Dybdahl1, Chenxi Huang1
1 Department of Radiology, Copenhagen University Hospital, Rigshospitalet
* Correspondence: marco.dybdahl@regionh.dk
Clinical history
A 44-year-old male with a 3-year history of ulcerative colitis (UC) and primary sclerosing cholangitis (PSC) was diagnosed with acute myeloid leukemia (AML). He started immunosuppressive treatment with FLAG-IDA (fludarabine, cytarabine, granulocyte colony stimulating factor and idarubicin) and was scheduled for a hematopoietic stem cell transplantation (HSCT). Two weeks after initiation of the FLAG-IDA treatment, the patient was admitted to hospital with neutropenic fever, jaundice and diarrhea. Initial blood samples showed normal liver function tests, but signs of acute renal failure, pancytopenia, high plasma-lactate and plasma-CRP indicated sepsis with some degree of organ failure. The patient was transferred to the intensive care unit due to septic shock and a suspicion of progressive liver failure. Blood cultures were positive for clostridium perfringens.
The patient was treated with broad spectrum antibiotics and antiviral medication, due to the induced pancytopenia and the suspicion of widespread infection and sepsis. Corticosteroids were added, as there was also a suspicion of activity in the patient’s UC.
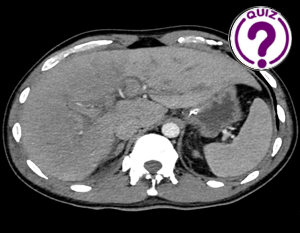
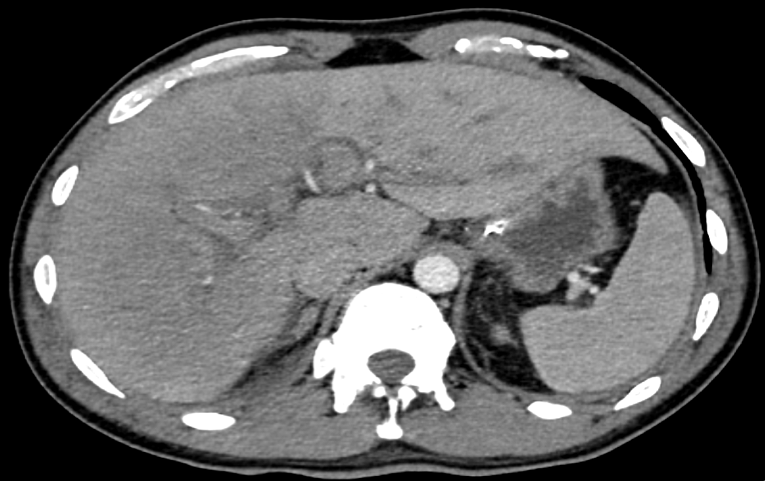
A CT (Computed tomography) scan of the thorax and abdomen with IV-contrast was requested.
Image
Quiz-summary
0 of 4 questions completed
Questions:
- 1
- 2
- 3
- 4
Information
View the August Case below, answer the question and then click check >
You have already completed the quiz before. Hence you can not start it again.
Quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Results
0 of 4 questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 points, (0)
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- Answered
- Review
-
Question 1 of 4
1. Question
Question 1: What findings are visible on the shown CT image?
Correct
CORRECT ANSWER EXPLAINED BELOW Correct answer to Q1 is: All of the above.
Incorrect
CORRECT ANSWER EXPLAINED BELOW Correct answer to Q1 is: All of the above.
-
Question 2 of 4
2. Question
Discussion:
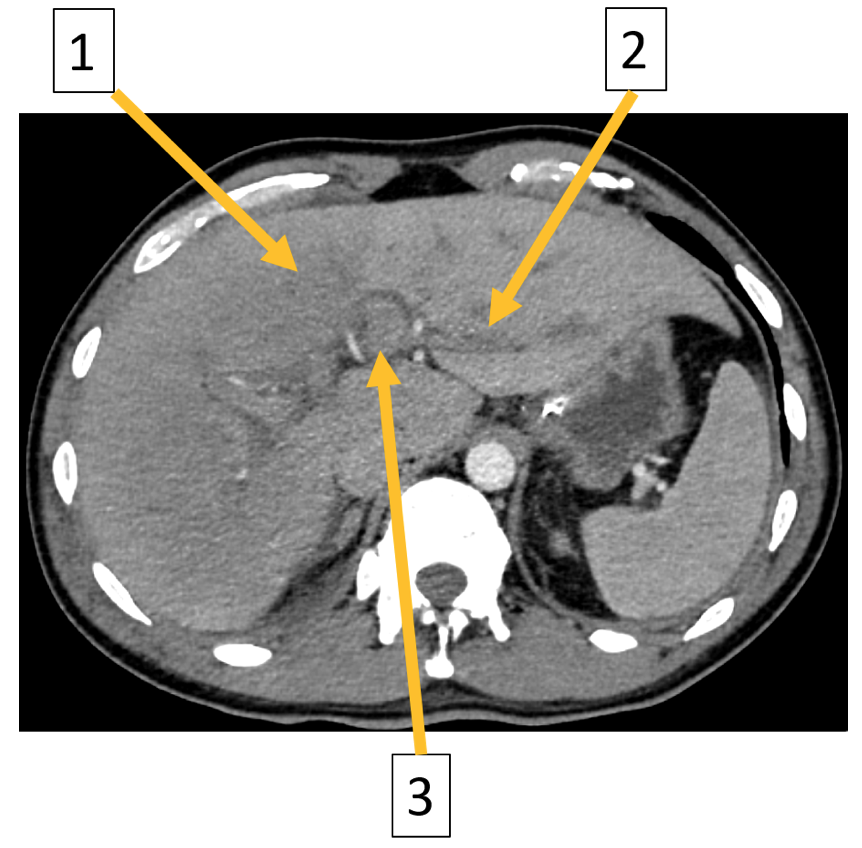
Image 2: Axial contrast enhanced CT of the abdomen in portal venous phase: (1) Reduced enhancement of the liver parenchyma, (2) periportal edema, and (3) reduced enhancement of the portal veins.
The CT showed reduced enhancement of the liver, most evident in the right liver lobe. Additionally, lower than expected venous enhancement was seen in the inferior vena cava, liver veins and the portal vein. Furthermore, there was a slight hepatomegaly, diffuse wall thickening of the gall bladder, ascites, dilatation of the intrahepatic bile ducts and periportal edema. This was attributed to either a reduced cardiac output or hepatic congestion.
Due to suspicion of hepatic congestion an ultrasound (US) examination was requested. The following image was acquired during the examination.
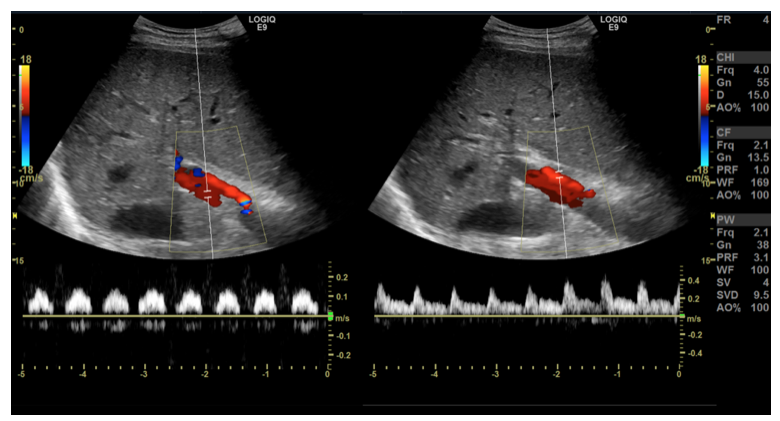
Image 3: Subcostal transverse view with color and spectral Doppler depicting the flow in the portal vein and the hepatic artery, respectively.
Question 2: Which of the following answers corresponds best with what you see on the US image?
Correct
CORRECT ANSWER EXPLAINED BELOW Correct answer to Q2 is: Abnormal, pulsatile flow in the portal vein and normal, arterial flow in the hepatic artery.
Incorrect
CORRECT ANSWER EXPLAINED BELOW Correct answer to Q2 is: Abnormal, pulsatile flow in the portal vein and normal, arterial flow in the hepatic artery.
-
Question 3 of 4
3. Question
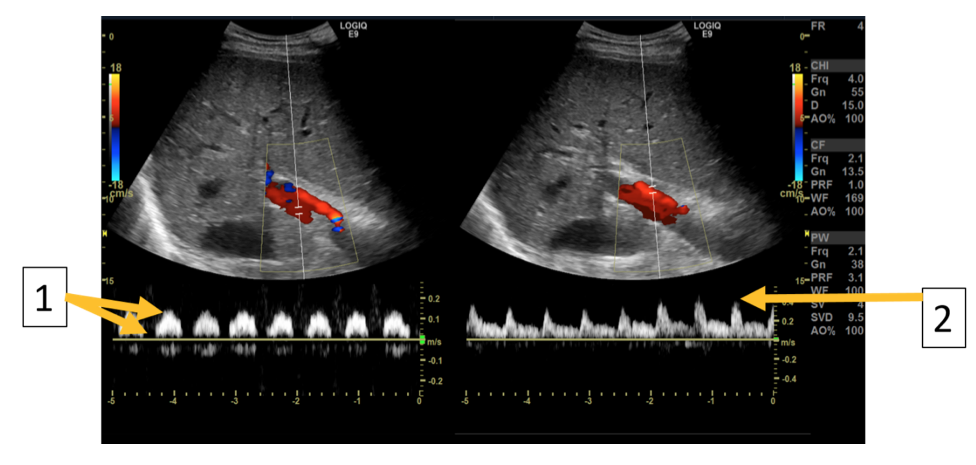
Image 4: Subcostal transverse view with color and spectral Doppler depicting the flow in the portal vein and the hepatic artery respectively. (1) The portal vein has abnormal, pulsatile flow, with “dips” in flow rate synchronous with pulsations of the hepatic artery. (2) The hepatic artery has normal arterial flow characteristics.
To make sure that there was no thrombus in the liver veins a supplemental contrast enhanced ultrasound examination (CEUS) was performed. Video/images are shown below.
Video 1: CEUS, subcostal transverse view, shortly after administration of 1.6 mL of SonoVue IV-contrast.
Video 2: CEUS, subcostal transverse view, 30-40 seconds after SonoVue administration.
Question 3: Which of the following answers corresponds best with what you see on the CEUS videos?
Correct
CORRECT ANSWER EXPLAINED BELOW Correct answer to Q3 is: Patent central portal veins and liver veins with no signs of thrombosis.
Incorrect
CORRECT ANSWER EXPLAINED BELOW Correct answer to Q3 is: Patent central portal veins and liver veins with no signs of thrombosis.
-
Question 4 of 4
4. Question
The first CEUS-video shows normal contrast enhancement of the inferior vena cava and the hepatic veins; however, no enhancement of the portal vein is seen at this time. In the second CEUS-video the contrast has reached the central portal veins with no signs of thrombosis.
Question 4: Which condition is most likely, considering the presented CEUS findings?
Correct
CORRECT ANSWER EXPLAINED BELOW Correct answer to Q4 is: Hepatic veno-occlusive disease (VOD)/Sinusoidal obstruction syndrome (SOS)
Discussion:
There was no evidence of thrombosis on neither the CT nor the CEUS examination, making Budd-Chiari syndrome highly unlikely. In drug-induced liver injury the time span is often shorter, and it is not specifically associated with hemodynamic changes in the liver veins/portal vein. There is no history available with regards to alcohol and drugs, but acute, toxic affection of the liver would show severe affection of the liver function blood tests and not necessarily impaired liver hemodynamics.
Sinusoidal obstruction syndrome (SOS) or hepatic veno-occlusive disease (VOD), is believed to result from toxic damage to the liver sinusoids from drugs used during the hematopoetic stem cell transplantation (1). This causes endothelial cells to slough off and obstruct hepatic venules and subsequently postsinusoidal obstruction ultimately resulting in hepatic congestion. According to the European society for blood and marrow transplantation, SOS is seen within 21 days from HSCT and requires the presence of elevated bilirubin and the presence of at least two of the following findings: painful hepatomegaly, weight gain and/or ascites (2). There is no established standard treatment, but available options include anticoagulation, vasodilators, Defibrotide, and supportive and intensive care. The prognosis of SOS depends on severity and treatment response, with early detection improving outcomes, however the risk of mortality is high in severe cases.
Conclusion
US and CEUS are often readily available problem-solving tools that should be considered in equivocal cases. US plays a critical role in diagnosing SOS by providing non-invasive imaging to assess liver morphology and detect signs of deteriorating liver function. Doppler ultrasound can quickly evaluate hepatic blood flow and identify signs of congestion or thrombosis. CEUS further improves detection of perfusion abnormalities and provides detailed vascular assessment. Together, these imaging techniques are vital for early and accurate identification of SOS, guiding effective patient management (3).
Conflicts of interest
“The authors declare no conflict of interest.”
References
- Bonifazi F, Barbato F, Ravaioli F, Sessa M, Defrancesco I, Arpinati M, Cavo M, Colecchia A. Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. fimmu. 2020 Apr 3;11:489.
- Mohty M, Malard F, Alaskar AS, Aljurf M, Arat M, Bader P, Baron F, Bazarbachi A, Blaise D, Brissot E, Ciceri F, Corbacioglu S, Dalle JH, Dignan F, Huynh A, Kenyon M, Nagler A, Pagliuca A, Perić Z, Richardson PG, Ruggeri A, Ruutu T, Yakoub-Agha I, Duarte RF, Carreras E. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a refined classification from the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant. 2023 Jul;58(7):749-754.
- “Sinusoidal obstruction syndrome” | Radiology Reference Article | Radiopaedia.org Available online: https://radiopaedia.org/articles/sinusoidal-obstruction-syndrome-1 [accessed on 31. July 2024].
Incorrect
CORRECT ANSWER EXPLAINED BELOW Correct answer to Q4 is: Hepatic veno-occlusive disease (VOD)/Sinusoidal obstruction syndrome (SOS)
Discussion:
There was no evidence of thrombosis on neither the CT nor the CEUS examination, making Budd-Chiari syndrome highly unlikely. In drug-induced liver injury the time span is often shorter, and it is not specifically associated with hemodynamic changes in the liver veins/portal vein. There is no history available with regards to alcohol and drugs, but acute, toxic affection of the liver would show severe affection of the liver function blood tests and not necessarily impaired liver hemodynamics.
Sinusoidal obstruction syndrome (SOS) or hepatic veno-occlusive disease (VOD), is believed to result from toxic damage to the liver sinusoids from drugs used during the hematopoetic stem cell transplantation (1). This causes endothelial cells to slough off and obstruct hepatic venules and subsequently postsinusoidal obstruction ultimately resulting in hepatic congestion. According to the European society for blood and marrow transplantation, SOS is seen within 21 days from HSCT and requires the presence of elevated bilirubin and the presence of at least two of the following findings: painful hepatomegaly, weight gain and/or ascites (2). There is no established standard treatment, but available options include anticoagulation, vasodilators, Defibrotide, and supportive and intensive care. The prognosis of SOS depends on severity and treatment response, with early detection improving outcomes, however the risk of mortality is high in severe cases.
Conclusion
US and CEUS are often readily available problem-solving tools that should be considered in equivocal cases. US plays a critical role in diagnosing SOS by providing non-invasive imaging to assess liver morphology and detect signs of deteriorating liver function. Doppler ultrasound can quickly evaluate hepatic blood flow and identify signs of congestion or thrombosis. CEUS further improves detection of perfusion abnormalities and provides detailed vascular assessment. Together, these imaging techniques are vital for early and accurate identification of SOS, guiding effective patient management (3).
Conflicts of interest
“The authors declare no conflict of interest.”
References
- Bonifazi F, Barbato F, Ravaioli F, Sessa M, Defrancesco I, Arpinati M, Cavo M, Colecchia A. Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. fimmu. 2020 Apr 3;11:489.
- Mohty M, Malard F, Alaskar AS, Aljurf M, Arat M, Bader P, Baron F, Bazarbachi A, Blaise D, Brissot E, Ciceri F, Corbacioglu S, Dalle JH, Dignan F, Huynh A, Kenyon M, Nagler A, Pagliuca A, Perić Z, Richardson PG, Ruggeri A, Ruutu T, Yakoub-Agha I, Duarte RF, Carreras E. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a refined classification from the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant. 2023 Jul;58(7):749-754.
- “Sinusoidal obstruction syndrome” | Radiology Reference Article | Radiopaedia.org Available online: https://radiopaedia.org/articles/sinusoidal-obstruction-syndrome-1 [accessed on 31. July 2024].