- WFUMB Stands with EFSUMB: stop bloodshed in Ukraine - read our statement here >
WIOTM
- All
- 25th May Pre Congress
- 26th May Day 1 Congress
- 28th May Day 3 Congress
- Administrative Councillors
- books
- Chairs
- Clinical Ultrasound
- COE Centre
- COE Task Force
- Collaboration Committee
- Committees
- Congress 2022
- Congress Committee
- Congress News
- Constitution Committee
- E-Book
- E-learning Task Force
- echoes
- Education Committee
- EXB
- Featured
- Featured_Congresses
- Featured_External Meetings
- Featured_Federation
- In Memoriam
- Investment Advisory Committee
- NEWS
- NEWS CoE's
- NEWS Partnerships
- Nominating Committee
- Pioneers
- Publications Committee
- Room 1 - Alfa 1
- Room 1 - Alfa 1
- Room 2 - Alfa 2
- Room 2 - Alfa 2
- Room 3 - Alfa 3
- Room 4 - Atena
- Room 4 - Atena
- Room 5 - Viena
- Room 6 - Roma 1
- Room 7 - Roma 2
- Room 8- Oslo
- Safety Committee
- Safety Statements
- Student Education
- Ultrasound the Best
- UMB Publications
- Uncategorized
- Webinars
- WIOTM
July 5, 2024
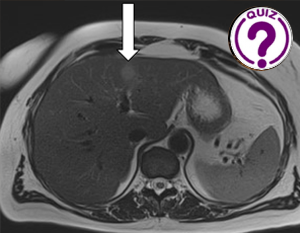
Yung-Liang Wan1,* Yao-Fan Fang2, Wen-Yu Chuang3, Kuang-Hui Yu2 1. Department of Medical Imaging and Intervention, Linkou Chang Gung Memorial Hospital, Chang Gung University, Taoyuan City, Taiwan […]
June 17, 2024
Rune Fuglø-Mortensen1*, Jonathan Frydenlund Cohen2 Department of Radiology, Herlev and Gentofte Hospital, Denmark; Department of Radiology, Rigshospitalet, Copenhagen University Hospital, Denmark * Correspondences: rune.fugloe.mortensen@regionh.dk Clinical history […]
May 28, 2024
Jeong Yeon Cho Department of Radiology, Seoul National University Hospital, Seoul, Korea * Correspondences: radjycho@snu.ac.kr Clinical history A 31-year-old pregnant woman was referred to our department at […]
March 30, 2024
Jihene Belhadj Ali, Wiém Douira-Khomsi Department of Paediatric Radiology, Béchir Hamza Children’s Hospital, Tunis, Tunisia * * Correspondences: bhjjihene@gmail.com ; khomsiwiem@yahoo.fr Clinical history A […]
March 15, 2024
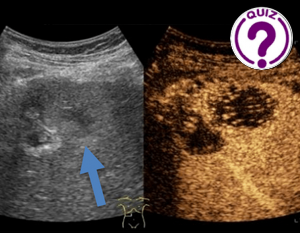
Maria Franca Meloni1 *, Debora Cidoni2 , Giacomo Gazzano1, and Sandro Sironi2 , 1 Casa di Cura Igea: Department of Radiology, Milano, Italy 2 Department of […]
March 1, 2024
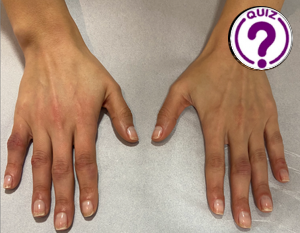
Eliza Ducati 1*and Isabella Wender 2 1 Radiology Department, Hospital das Clínicas São Paulo – São Paulo, Brazil. 2 Dermatology Department, Hospital São Lucas da PUCRS […]
February 1, 2024
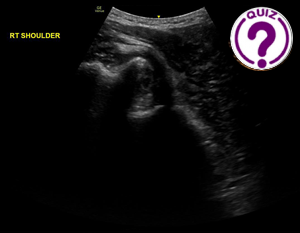
Adrian Goudie 1 1 Fiona Stanley Hospital, Perth, Australia. * Correspondence: adrian.goudie@health.wa.gov.au Clinical history A middle aged man presented with a painful right shoulder after falling […]
January 5, 2024
Camilla Palmquist Frykman1* and Chenxi Huang2 1 Department of Radiology, Herlev and Gentofte Hospital, Denmark; camilla.palmquist.frykman.@regionh.dk 2 Department of Radiology, Copenhagen University Hospital Rigshospitalet; chenxi.huang.01@regionh.dk * […]
November 29, 2023
Qingli Zhu1, Yuanjing Gao1, Yi-Hong Chou*2 1 Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China 2 Department […]